Abstract
INTRODUCTION
Multiple myeloma (MM) is an incurable neoplasm arising from mature plasma cells in the bone marrow. Different genetic aberrations including translocations and deletions have been described in MM especially involving immunoglobulin heavy chain locus in chromosome 14. Such translocations include t(4;14), t(14;16), and t(14;20) in addition to del13q and del1p32. These events are associated with poor prognosis and are under investigation for novel therapeutic approaches. Identification of these events can be detected by either metaphase cytogenetics or by fluorescent in situ hybridization (FISH). We aimed to study the use and reporting of cytogenetics in phase III clinical trials of MM.
METHODS
We performed a cross-sectional study by retrieving interventional MM phase III clinical trials from clincaltrials.gov. All phase III interventional MM clinical trials were included. NCT number, article title, publication date, initiation year, number of enrolled patients funding source, location, cytogenetics performed, and the percentage of unknown/missing cytogenetics were extracted. Descriptive statistics, the Mann-Whitney U test, and the Spearman correlation test were used to analyze the data. Statstical analysis was done with SPSS® Statistics.
RESULTS
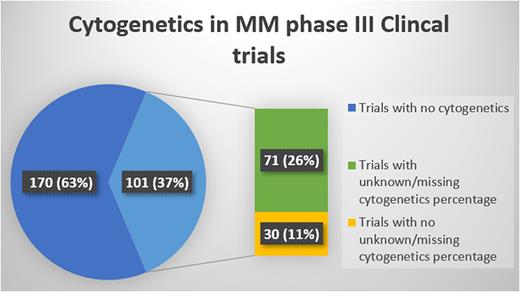
Of 271 phase III clinical trials, 101 studies performed cytogenetics testing. Full-text publications with results were available for 87% of those studies (n=88/101). The total number of patients enrolled was 53,411 with a median number of 455 (IQR= 430) per trial. The median publication year was 2017 (IQR= 8) and the median study initiation year was 2011 (IQR=9). del(17p) was the most commonly performed and/or reported cytogenetic abnormality in 93 trials (92%), followed by t(4;14) in 90 trials (89%), t(14;16) in 66 trials (63%), 1q gain in 15 trials (15%), del13q in 14 trials (14%), and t(14;20) in 8 trials (8%). Missing cytogenetics data were reported in 71 trials and a total of 9831 patients with a median of 23.37% (IQR= 38.33%) missing cytogenetic results per trial. [Figure.1] The later the initiation date of the trial significantly correlated with a lower missing cytogenetics percentage (p-value <0.05). The number of enrolled patients, trial location, and funding type did not correlate with missing cytogenetics percentage.
CONCLUSIONS
Less than half of MM trials reported the use of cytogenetics in testing for enrolled patients. When reported approximately 1 of 4 enrolled patients had an unknown/missing cytogenetics profile. A trend of improvement indicated by lower percentage of missing cytogenetics testing was noted in recently conducted clinical trials. Efforts should be made to minimize missing cytogenetics data in enrolled MM patient in clinical trials.
Disclosures
Schinke:Janssen: Honoraria. van Rhee:GlaxoSmithKline: Consultancy; Karyopharm: Consultancy; Takeda: Consultancy; Janssen Pharmaceuticals: Research Funding; Bristol Myers Squibb: Research Funding; EUSA Pharma: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal